Scientific literature:
Pulpotec® – a clinically studied material
For more than 20 years, Pulpotec® has been used worldwide. It is estimated that more than 25 million pulpotomy treatments were performed with Pulpotec®.
The historical efficiency and safety of Pulpotec® is recognised by a wide range of international dentists that have experimented pulpotomy procedure on both primary and permanent teeth with Pulpotec®. Our post-market surveillance does not highlight adverse events of concern regarding the safety of the product. On the other hand, the efficiency on the long term is confirmed with some clinical and radiographical follow-up covering period up to 18 years.
Regarding scientific literature, Pulpotec® is well documented.
It includes:
- More than 30 published articles in international paper;
- 1’142 teeth treated with Pulpotec® as part of clinical trials. The clinical trials are mainly comparative studies with MTA and Formocresol based on clinical and radiographical outcomes;
- 3’089 teeth treated with Pulpotec® as part of documented clinical follow-up in dental office.
The conclusion and scope of each single article including data on Pulpotec® are given hereafter. You can download most of these articles to explore the details of these clinical trials.
The comparative clinical trials mainly focused on Pulpotec®, MTA and Formocresol. The highlighted range of value of clinical success rate and radiographical success rate is given in the following table. With only one exception, the differences were statistically not significant.
These success rates are consistent with other clinical trials on MTA and Formocresol encountered in literature. The value obtained for other pulpotomy materials are lower or similar: 89%-100% clinical success rate and 86%-97% radiographic success rate for Ferric Sulfate; 80%-88% radiographical success rate for Calcium hydroxide.
| Pulpotec | Formocresol | MTA | |
|---|---|---|---|
| Clinical success rate | 93.3% - 100% | 73.3% - 100% | 97% - 100% |
| Radiographical success rate | 83% - 100% | 78.6% - 96.7% | 91.2% - 94% |
The common conclusion of these studies is that Pulpotec® and MTA can successfully eliminate side effects associated with Formocresol and be considered as recommended pulpotomy material with high clinical and radiographical success rate. The authors also highlighted the paramount importance of the tightness of coronal seal on the success rate of the treatment.
Regarding other outcomes, the management of pain is especially highlighted for Pulpotec®: 80% to 100% of patient have immediate pain relief after pulpotomy treatment with Pulpotec®. In the minority of case where pain remains, it is assessed by the patient as mild pain and it disappears in most cases within 2-3 days.
Signs of pathology (change of periapical tissues, internal or external root resorption, swelling, etc.) are in average present in less than 6% for treatment performed with Pulpotec®. This is similar to observations with MTA. In average, Formocresol, Ferric sulfate and Calcium hydroxide show higher level of signs of pathology.
Available articles related to Pulpotec®
Randomized follow-up at 3 and 6 months / 60 primary molars/ 43 children / aged 7-9 years
Clinical and histopathological evaluations at 6 months showed that Pulpotec® produces more favorable outcomes than Formocresol and Ferric sulfate. It was found that Pulpotec® induce less clinical findings (pain, gingival swelling, mobility tenderness to percussion, fistula) and less histopathological findings (mild inflammation, severe inflammation, necrosis, abscess) than both Formocresol and Ferric sulfate. The results were statistically significant. The authors concludes that Pulpotec® could successfully eliminate side effects associated with Formocresol and Ferric Sulfate in pulpotomy procedure for primary teeth.
Source: M.A. Karrem, A clinical and histopathological evaluation of different pulpotomy agents in primary teeth, IASJ, 2012
Follow-up at 1-, 3- and 6-months / 45 primary molars / 39 children
After six months, the clinical success rate of Pulpotec® was 93.3%, Formocresol 73.3% and MTA 100%. The differences were statistically not significant. In Pulpotec® group, absence of pain at all patients without exception was observed. No complaints were lodged by patients either in the intervals between visits to clinic or during the dynamic observation. After 6 months follow-up, no swelling of gum in the area of the treated tooth, no evidence of a fistula and no mobility of a tooth, were observed. The authors concludes that Pulpotec® and MTA could successfully eliminate side effects associated with Formocresol and Ferric Sulfate in pulpotomy procedure for primary teeth.
Source: A.A. Al-Dahan et al., Clinical and radiographical evaluation of pulpotomy in primary molars treated with Pulpotec® (PD), Formocresol and Mineral Trioxide Aggregate (MTA), J Bagh Colllege Dentistry, 164-170, 2013
Follow-up at 3- and 6-months / 90 primary molars / 62 children / aged 4-9 years
At the end of 6 months, clinical success was 100% for all the three groups. Whereas radiographic success was 96.7% for Formocresol and 100 % for both Pulpotec® and Biodentine group. The differences were statistically not significant. The authors concludes that Pulpotec® and Biodentine could be considered alternative to Formocresol as pulpotomy agent.
Source: B. Verma et al, Comparative evaluation of success of pulpotomy in primary molars treated with formocresol, Pulpotec® and biodentine – 6 month follow up study, IJADS, 77-82, 2019
Randomized follow-up at 6-, 12-,18- and 24-months / 105 primary molars / 58 children / aged 3-9 years
After 24 months, clinical efficiency of pulpotomy was high in all groups (97%). Radiographic efficiency at 24-months was 94.3% for Pulpotec®, 91.2% for MTA, 83.3% for Formocresol. According to radiography the treatment outcomes of Pulpotec® use were higher compared with MTA and formocresaol but without statistical significance. Natural exfoliation occurs during the clinical trials. The examination revealed corresponding erupted permanent teeth without any abnormality in their structure. Clinical examination of the children after 18 and 24 months revealed no complaints in children; gingiva in the area of the treated teeth had normal color and consistency, percussion and palpation were painless. Insignificant darkening of treated teeth crowns was noticed in the MTA group (60.6% of the cases). After 24 months the signs of pathology on x-rays (periodontal ligament space increase, internal or external root resorption, and the increase of radiographic radiolucencies in furcation area) were revealed in the Pulpotec® group in 5.7% cases, in the MTA group – 8.8% cases, in the Formocresol group – 16.7% cases.
Source: E.E. Maslak et al., Pulpotomy efficiency in primary molars: outcomes of 24-month randomized clinical trial, 2020
Follow-up at 3-, 6- and 9-months / 90 primary molars / 30 children / aged 4-8 years
At end of 9 months, Pulpotec® and MTA showed 100% of clinical success rate, when Formocresol showed 92.9% success rate (no statistically significant difference). Regarding the radiographic examinations, Pulpotec® showed the highest radiographic success rate (100%) in comparison to MTA (92.9%) and Formocresol (78.6%) (with statistically significant difference). In the Pulpotec® group, there were absence of pain, swelling, fistula and mobility at all patient without exception after use of Pulpotec®. The authors conclude that Pulpotec® and MTA are materials with high clinical and radiographic success rate and could be used as pulpotomy materials.
Source: O. B. Zewail et al., Comparative study of the clinical and radiographic effects of Pulpotec® and mineral trioxide aggregate on the pulp of the primary molars, Tanta Dental Journal, 9-14, 2020
60 teeth / 60 patients / aged 20-35 years
Pulpotec® shows complete relief of pain (100%), while CMPC and Eugenol show mild to severe pain after one to three days. The authors concludes that the constituents of Pulpotec® provide alleviation of pain in symptomatic teeth due to their anti-inflammatory effect and reduce the side effect. That will diminish the use of systemic antibiotics.
Source: Z. M. Mansi et al., The effect of three different dressing materials on pain relief of symptomatic teeth. Randomized clinical trial, MDJ, 8-14, 2019
Follow-up at 2-, 4- and 6-months / 30 teeth / 30 children / aged from 4-7 years
Immediate pain relief after treatment was observed in 80% of cases; mild pain which lasts only 2 to 3 days in 20% of cases. The teeth are clinically mute and function normally. Clinical and radiographical examinations carried out on follow up visits revealed that all cases showed a healthy physiological image with no trace of any pathological changes. Concerning the treated immature permanent molars, there was clear evidence of continued root formation that was observed radiographically in the follow up visits. The authors concludes that positive results of clinical trials of Pulpotec® preparation enable to recommend it for use in extensive clinical practice.
Source: K.A. Al-Salman et al., The effectiveness of using Pulpotec® in treatment of pulpitis by pulpotomy of vital deciduous molar and vital immature permanent molar, 185-190, 2012
6 to 17 weeks follow-up / 42 teeth / 30 children
Pulpotec® showed 100 % success rate, Formocresol showed 90.90%. No statistically significant difference was observed between the two groups. The authors concludes that Pulpotec® can handle inflammation better than Formocresol. It may be due to the corticosteroid content which is well-known for its anti-inflammatory effect.
Source: A. T. Htun et al., Clinical evaluation of formocresol and iodoform-corticosteroid containing medicaments used for pulpotomies of deciduous molars, 2007
Follow-up at 6-, 12-, 18- and 24-months / 84 teeth / 21 children
Clinical evaluation at 24 months shows success rate of 94% for Pulpotec®, 94% for Formocresol, 100% for MTA, 83% for Emdogain. Radiographic evaluation at 24 months shows success rate of 83% for Pulpotec®, 88% for Formocresol, 94% for MTA and 72% for Emdogain. The differences were statistically not significant. The authors concludes that Pulpotec® as well as MTA and Emdogain could be considered alternative to Formocresol as pulpotomy agent. It is noted that Pulpotec® has an advantage of having clinical success even in cases with a little residual blood in the pulp chamber when used as a pulpotomy medicament.
Source: B. Sunitha et al., Clinical and Radiographic Evaluation of Four Different Pulpotomy Agents in Primary Molars: A Longitudinal Study, IJCPD, 240-244, 2017
Follow-up at 12-months / 40 primary necrotic molars / 40 children / aged 4-6 years
In this study 67.7% of the patients showed healing of bone loss, both in height and width directions (80.6%, 71%). The pain, swelling and fistula disappeared in all except one case after one week. At the follow-up no clinical pathological signs were detected, even in teeth with radiological failure. The authors concludes that the use of Pulpotec® may reduce the clinical signs of infection in necrotic molars, and preserve the tooth on the arch for a longer time as in the case before the eruption of the first permanent molar.
Source: S. Aboujaoude et al., Evaluation of a modified Pulpotec® endodontic approach on necrotic primary molars: a one-year follow-up, European Journal of Paediatric Dentistry, 111-114, 2015
860 teeth / 860 patient
The incidence of intense pain was 1.16% and 0.69% of the treatment cases at 24 and 48 h, respectively. At 7 days, all patients experienced no pain or only weak pain levels. The authors conclude that Pulpotec® has a beneficial impact on pain and/or swelling in emergency root canal treatment and in controlling postoperative pain in multi-appointment root canal treatment.
Source: B. Faraj, Four years of clinical experience with the efficacy of Pulpotec® as a root canal dressing for the management and control of odontogenic pain: a prospective randomized clinical trial, OHDM, 279-283, 2013
Follow-up at 24-months / 40 teeth / 20 children / aged 7-10 years
The authors concludes that collagen and Pulpotec® are materials neither toxic nor biologically incompatible that can be used as pulpotomy material. Collagen is found to be a better alternative to Pulpotec®.
Source: P. Kakarla et al., Dental pulp response to collagen and Pulpotec® cement as pulpotomy agents in primary dentition: A histological study, J Consev Dent, 434-438, 2013
1’500 primary molars and 1’500 permanent molars / 17 years of utilization in dental office
For treatment of primary teeth, immediate pain relief occurs after treatment in 80% of cases; mild pain lasts only 2 to 3 days in 20% of cases. These teeth are clinically mute and function until they disappear from the dental arch to give way to the permanent teeth which follow them. The follow-up of immature permanent molars treated with Pulpotec®, has indicated normal, progressive radicular edification until complete maturity. For treatment of permanent teeth, the documented X-Ray cases present a healthy physiological image with no trace of periapical infection in the follow-up X-ray, added to which, these teeth were not painful and mastication was perfectly normal.
Source: Final report of clinical trials of Pulpotec®, dental office clinical follow-up, 2002
42 teeth / 42 patients (37 children, 5 adults) / aged 4-56 years
Clinical follow-up provided have shown absence of pains at all patients without exception after use of Pulpotec®. Even if the pain syndrome was found evident at diagnostics of odontitis it was completely arrested after depositing of the first portion of the preparation. No complaints were lodged by patients either in the intervals between visits to clinic or during the dynamic observation (from 4 to 6 months). No swelling of gum in the area of the treated tooth was detected during the given period, no evidence of a fistula and no mobility of a tooth.
Source: S.A. Dedeyan et al., Use of Pulpotec® for treatment of odontitis in pediatrics, 2003
18 teeth / 16 patients
For 83.3% of the patient, sensation of pain disappeared immediately after treatment procedure and for 17.7% mild pain is present only for the next day.
Source: S. Melekhov et al., Treatment of the multirooted teeth by the amputation method using Pulpotec®, The dentistry today, 17, 2002
27 molars / 27 patients / aged from 11 to 54 years
X-rays were taken prior to treatment, and no pathological changes were noted in any of the treated patients. The data gathered at 6 months and 1 year revealed no changes in the periapical tissues in 96.2% of the patients. Changes were noted in one patient who had reported pain when biting down on the treated tooth.
Source: S. Melekhov et al., Clinical experiment into the application of dental preparations for the treatment of pulpitis by the vital pulpotomy method, 27-33, 2008
27 molars / 27 patients / aged 17-54 years
After one year follow-up, 96% of patient treated with Pulpotec® show clinical and radiographical success.
Source: S. Melekhov et al., Comparative characteristics of modern preparations for the treatment of the pulp by the method of vital amputation, 57-59, 2008
X-Ray clinical cases
Since more than 20 years, Pulpotec® was used worldwide. It is estimated that more than 25 million pulpotomy treatments were performed with Pulpotec®.
From these numerus clinical cases, some X-Ray cases are presented hereafter.
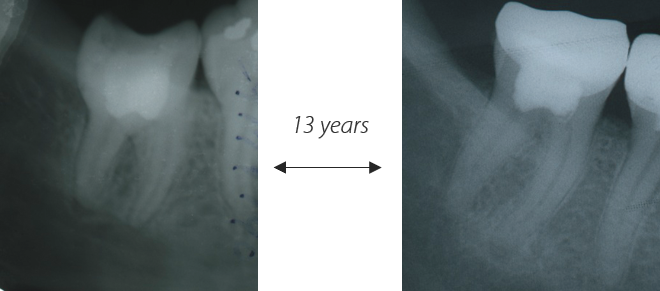
Figure 1 : Long term follow-up of pulpotomy with Pulpotec. Left – initial x-ray of tooth n°47. Right – x-ray control after 13 years
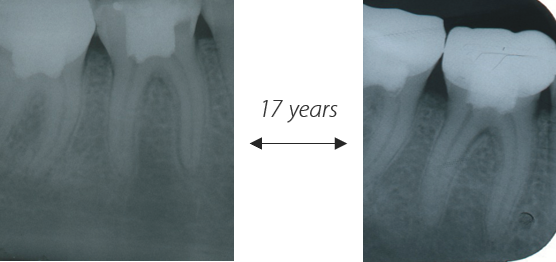
Figure 3 : Long term follow-up of pulpotomy with Pulpotec. Left – initial x-ray of tooth n°46. Right – x-ray control after 17 years
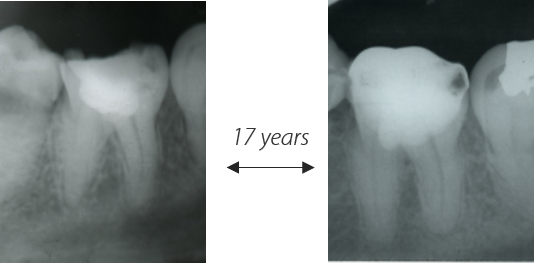
Figure 2 : Long term follow-up of pulpotomy with Pulpotec. Left – initial x-ray. Right – x-ray control after 17 years
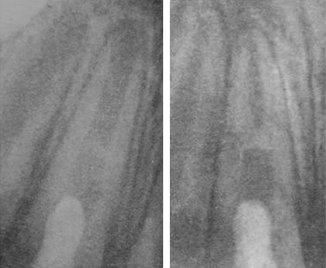
Figure 4 : Clinical case by Dr. Elena Satygo. Result of treatment with Pulpotec® after vital pulpotomy of an immature tooth which has undergone a traumatism. Left – X-Ray immediately after the treatment; Right – X-Ray after 1 year, highlighting complete apexification
Safety & formaldehyde
What is the state-of-the-art of formaldehyde in dentistry?
In year 2004, the International Agency for Research on Cancer (IACR) classified formaldehyde, one of the components of formocresol as carcinogenic to humans. However, the National Institute for Occupational Safety and Health in the United States and the Organization for Economic Cooperation and Development have since stated that “formaldehyde is not likely to be a potent carcinogen to humans when used under low exposure conditions.”
Hereafter we analyse with some key questions the state-of-the-art data on formaldehyde in dentistry based on scientific literature (B. Athanassiadis et al., A review of the effects of formaldehyde release from endodontic materials, International Endodontic Journal, 829-838, 2015).
Formaldehyde is a naturally occurring organic compound with the formula CH2O. Formaldehyde is naturally present in most living cells and the environment.
Everyone is exposed to formaldehyde on a daily basis from various sources related to lifestyle and diet. Some of these sources are foods such as shiitake mushrooms (40–380 ppm), fresh seafoods (2 ppm) or fruit and vegetables (3–22 ppm). Inhalation of trace amounts of formaldehyde can easily occur from multiple sources, such as the decomposition of plant residues, automotive exhaust, cigarette smoke, outgassing of furniture, insulating materials used in construction, workplace use of various synthetic resins and glues, fabrics, cosmetics and hair straightening products. Combining these various sources, the World Health Organization (WHO) estimated daily intake of formaldehyde for an adult is about 10.55 mg/day, comprising 9.4 mg/day from food, 1 mg/day from inhalation and 0.15 mg/day from water.
The possible routes of exposure to formaldehyde are by ingestion, inhalation, dermal absorption and blood exchange. Once absorbed, formaldehyde is very quickly broken down. Almost every tissue in the body has the ability to breakdown formaldehyde. It is usually converted to a nontoxic chemical called formate, which is excreted in the urine. Formaldehyde can also be converted to carbon dioxide and breathed out of the body.
Exogenous formaldehyde does not accumulate in the body as it has a biological half-life of only 1–1.5 min, and it is quickly cleared from human plasma. Such rapid metabolism would inhibit the systemic distribution of formaldehyde.
In the blood, the mean formaldehyde concentration is reported as 2.24 mg ± 0.07 mg/kg. WHO estimates that an adult is exposed to 10.55 mg formaldehyde per day from food, air and water. Based on these data, endogenous turnover of formaldehyde was estimated to be approximately 878– 1310 mg/kg body weight per day, assuming a half-life of 1–1.5 min.
As a consequence, it is not the presence of formaldehyde which is harmful, but the dose. It can be highlighted that adverse effects occur only at the point of contact after the concentration achieved is in excess of endogenous levels, and it exceeds the body’s ability to maintain homoeostasis. In those cases, formaldehyde is irritating to tissues when it comes into direct contact with them. It can destroy the skin’s protective oils causing dryness, cracking and dermatitis.
In dentistry, patients may be exposed to formaldehyde either during formocresol pulpotomies, or through materials that contain formaldehyde (such as N2 (Indrag-Agsa), Endomethasone (Septodont) or Pulpotec® (Produits Dentaires)) or materials that do not contain directly formaldehyde as an ingredient, but that release minimal levels of formaldehyde during their setting reaction (such as AH26 and AH Plus (Dentsply)).
When performing a pulpotomy, the mean dose of formocresol has been determined to be 0.013 mg per treatment, but the actual dose that interacts with the pulp is probably smaller than this. This amount is 1/810 of the 10.55 mg/day of formaldehyde that occurs in our daily intake from food, water and air. Note that in this case, formaldehyde present in formocresol is in liquid form, leading a higher availability than for formaldehyde present in paste form, such as for Pulpotec®. The value given here can be considered as a worst case. In conclusion, it appears that the amount of formaldehyde released during pulpotomies is less than the normal endogenous levels in humans, and they do not pose any health risks.
Pulpotomy treatment with Pulpotec® is less than 0.12% of the daily amount of formaldehyde that occurs in our daily intake from food, water and air (endogenous level) and that is efficiently and naturally eliminate by our body without accumulation.
In a review of all cohort studies published to February 2007 noted that industry workers and professionals exposed to formaldehyde showed no appreciable excess risk of cancers of the oral cavity, pharynx, sinus, nasal cavity and lungs.
However, appropriate personal protective equipment and good management of work area are highly recommended. These good behaviours are valid for both single and repeated exposure.
Regarding the safety of the practitioner or its assistant, it is recommended to wear gloves, mask and protectives glasses to avoid undesired contact with soft tissues. In the same logics, the use of a rubber dam is recommended for the patient. In case of contact, refer to the warning given in the Instructions For User. Precaution and good management of work area consists to regularly ventilate work area and to close immediately the powder and liquid vials after use.
As for single exposure, harm occurs in repeated exposure only at the point of contact after the concentration achieved is in excess of endogenous levels, and it exceeds the body’s ability to maintain homoeostasis.
Basics on pulpotomy
Pulpotomy is a procedure that consists to remove partially the pulp in vital tooth, as a mean of preserving the vitality and function of the remaining part. This vital pulp therapy (VPT) approach is in adequation with modern dentistry and related to minimally invasive procedure.
Pulpotomy approach is based on the capability of the pulp tissues to heal and regenerate. During the procedure, the portion of the pulp tissue that has undergone irreversible changes is removed from the coronal cavity of the tooth, leaving behind healthy and vital pulp tissue. This latter is dressed with material that maintains the survival of radicular pulp vitality and promotes repair. Tight crown restoration is then essential to ensure the durability of the pulp treatment.
The objective of every treatment should be, if possible, to maintain the vitality of the pulp of a tooth affected by caries, traumatic injury or other causes. Pulp vitality and function are important for both primary teeth and permanent (immature and mature) teeth.
“The vital pulp is by far the best canal obturation”
Prof. Marmasse
Historically, pulpotomy has been mainly focalized on primary teeth. A high proportion of deep carious lesions in primary teeth is associated with pulpal exposure. Pulpotomy is considered as the treatment of choice for infected coronal pulp in primary teeth, when the radicular pulp tissue is healthy or is capable of healing after removal of the infected part. The preservation of primary tooth in a functional state until they are replaced by the underlying permanent teeth is essential for maintenance of arch length, mastication, speech, esthetics and prevention of abnormal oral habits.
The advantage of pulpotomy is however not limited to primary teeth. In immature teeth, root development is required if possible. According, preservation of pulp vitality is very important. Pulpotomy allows the preservation and maintenance of pulp vitality, the continuation of root formation, leading to apical closure, and the development of valuable periodontal membrane and stronger structural resistance. Pulpotomy on mature permanent tooth was historically only performed in emergency procedure before root canal treatment (RCT). With new materials, pulpotomy is becoming a valid approach also for vital mature permanent tooth. By keeping the pulp functions alive, the dentin-pulp complex continues to protect itself by stimulating the formation of tertiary dentin or mineralized barrier against aggressions. Moreover, pulpotomy is technically less complicated, less time-consuming, and less expensive than RCT.
The choice of appropriate treatment depends on whether the pulp is vital or non-vital, based on the clinical diagnosis of normal pulp, reversible pulpitis, symptomatic or asymptomatic irreversible pulpitis, or necrotic pulp. In addition to clinical and radiological assessment, pulp vitality test is recommended to diagnosis the condition of the pulp. The most used techniques are cold / hot pulp testing, electric pulp testing, laser flowmetry or pulse oximetry.
The standard indications and contraindications for pulpotomy are listed hereafter.
Indications for pulpotomy:
- Tooth has no history of spontaneous pain
- Tooth has acute minor pain that subsides with analgesics
- Tooth has no discomfort to percussion, no vestibular swelling and no mobility
- Radiographic examination shows normal appearance of periodontal attachment
- Pulp is exposed during caries removal or subsequent to recent trauma
- Tissue appears vital
- Bleeding from the pulp excision site stops with isotonic saline irrigation within 2 minutes.
Contraindications for pulpotomy:
- A history of spontaneous toothache (not caused by papillitis resulting from food impaction)
- A non-restorable tooth where post-pulpotomy coronal seal would be inadequate
- A tooth near to exfoliation or if no bone overlies the crown of the permanent successor tooth
- Evidence of periapical or furcal pathosis
- Evidence of pathologic root resorption
- A pulp that does not bleed (necrotic)
- Inability to control radicular pulp hemorrhage following a coronal pulp amputation
- A pulp with serous or purulent drainage
- Presence of a sinus tract
The ideal material for pulpotomy should be bactericidal, harmless to the pulp and surrounding structures, promote healing of the radicular pulp, and not interfering with the physiological process of root resorption. However, the ideal material for pulpotomy has not yet been identified and there is no scientifical and clinical consensus on the best material to use for pulpotomy.
Several materials have been recommended as pulp medicaments following pulpotomy.
For over 80 years, Formocresol (FC) has been considered as an acceptable and the most commonly used material in primary teeth. Formocresol is still used for deciduous teeth with great success, even in cases of pulp necrosis. Currently commercial formocresol preparations consist of formaldehyde, cresol, glycerin and water. The product acts through the devitalization of the tissues in contact, making formocreasol especially efficient due to its bactericidal and fixative property. The use of formocresol is supported by several studies, which report clinical success rates between 70% and 98% in primary molars. Despite the high clinical and radiographic success rate, the use of formocresol has posed numerous questions, including its mutagenic potential, carcinogenic potential and local pulpal inflammation.
In year 2004, the International Agency for Research on Cancer (IACR) classified formaldehyde, one of the components of formocresol as carcinogenic to humans. However, the National Institute for Occupational Safety and Health in the United States and the Organization for Economic Cooperation and Development have since stated that “formaldehyde is not likely to be a potent carcinogen to humans when used under low exposure conditions.”
Regarding to these concerns, new pulpotomy materials have been introduced. The main alternatives are briefly described hereafter :
- Ferric sulfate (FS) is a haemostatic medicament. It minimizes the chances for inflammation and thereby prevent internal resorption. The response of blood to ferric sulphate ions induces agglutination of blood proteins forming a metal protein complex, which is capable of occluding capillaries and causing hemostasis.
- Zinc Oxide Eugenol (ZOE) can be used as preservative pulpotomy material. It possesses antimicrobial and anti-inflammatory properties. Nevertheless, internal resorption is associated with the use of ZOE and is a major drawback of this material for pulpotomy purpose.
- Calcium hydroxide (CH) is a highly alkaline material (pH > 12) that has bactericidal effect and can induce dentin bridge formation. The stimulation that this compound evokes is delicately situated between one of resorption and one of repair. Internal resorption is then the key drawback to this alternative action.
- Mineral trioxide aggregate (MTA), is a mineral cement mainly composed of calcium silicate and calcium aluminate. MTA is known thanks to its excellent physical characteristics, good sealing ability and biocompatibility. In addition, it forms thick and localized dentinal bridge, and promotes tissue regeneration. As for calcium hydroxide, the bactericidal effect is due to the alkalinity of the material (pH >12). The cost and difficulty in handling are the main drawbacks of this material.
- Pulpotec® is a paste with pharmacological constituents, including antiseptics and anti-inflammatory actions. It induces cicatrization at the product/pulp interface, while maintaining the vitality of the underlying radicular pulp.
To summarise, Formocresol pulpotomy benefits from good clinical and radiographic success rates, and is still a popular pulpotomy material despite the concerns raised due to its toxicity, mutagenicity and carcinogenicity. Alternatives exist and are widely clinically studied. This leads to clinical, radiographical and histopathological comparison between pulpotomy materials and highlight of advantages and limitation of each type of material in the scientific and clinical literature.

